Menu
- Reagents
- In-vitro assays
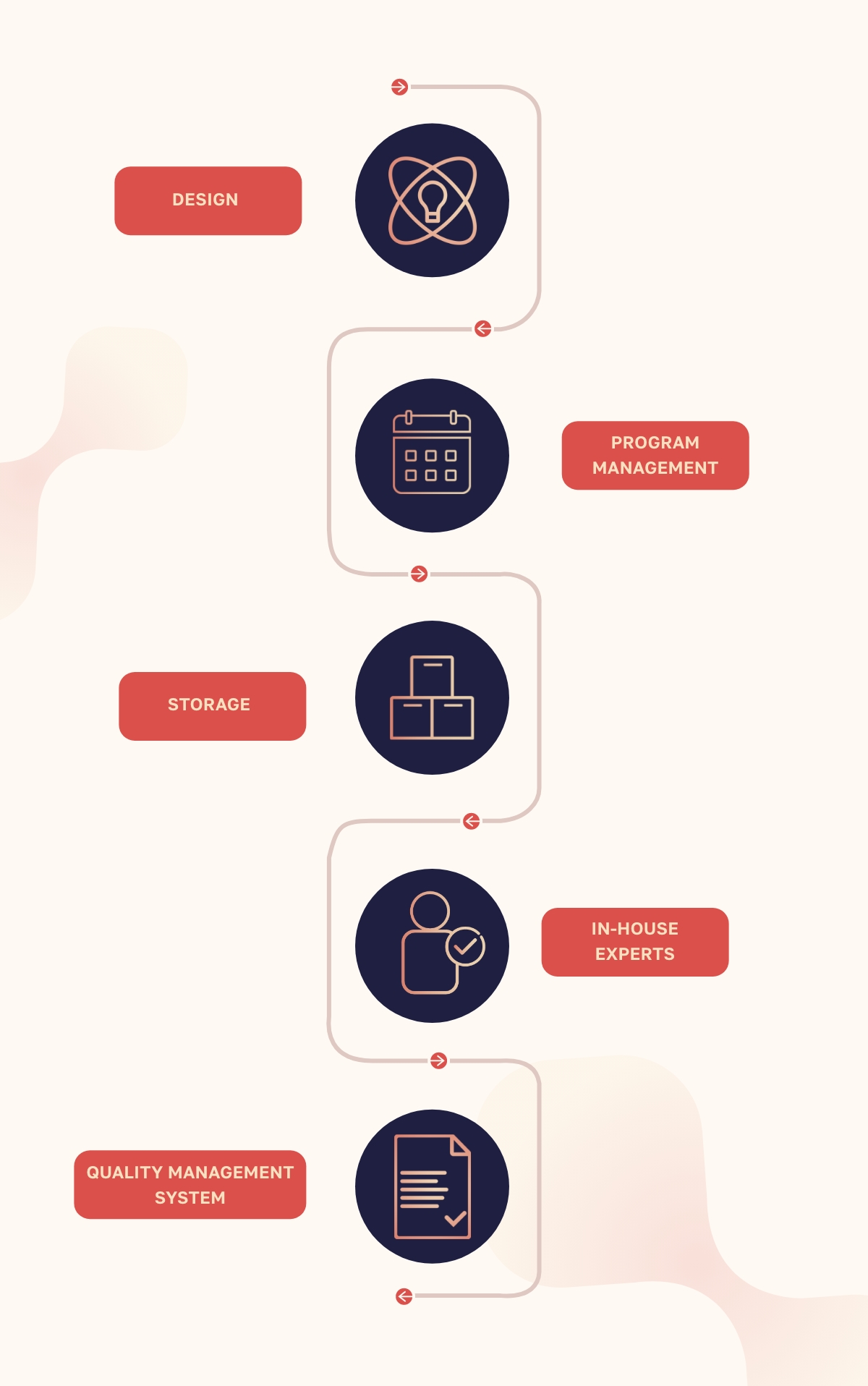
IN-VITRO Services
 In vitro assays are experiments conducted outside of a living organism, typically using isolated cells, tissues, or biochemical components.Menu
In vitro assays are experiments conducted outside of a living organism, typically using isolated cells, tissues, or biochemical components.Menu - Animal models
ANIMAL SERVICES
An animal model is a non-human species used in biomedical research because it can mimic aspects of a biological process or disease found in humans.
Menu - By Diseases targets
By Diseases Target
Disease-to-target discovery is the process of identification and early validation of targets involved in a disease.
Menu - Concierge Services
- Start an order